Citrus and Citrus Products
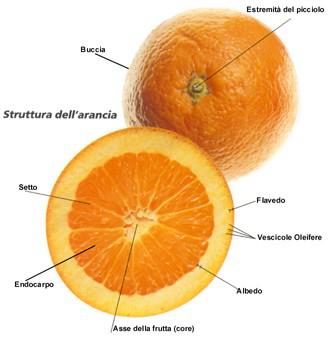
Citrus Structure
Citrus fruits have a complex structure. Regardless from cultivars, all citrus are similar except for shape and size. Lemons are oblong with major axis from stem to stylar, mandarins tend to be flat (oblate) with the major axis in the equatorial plane, perpendicular to the stem - stylar axis; most oranges tend to be round. Different are, also, sizes usually expressed as equatorial diameter (lemons from 4,4 to 6,4 cm , oranges from 5,7 to 9,5 cm ,grapefruits from 9,5 to 14,5 cm). These differences in fruit size between species and sometimes within cultivars within the same species require different sizing of equipment for juicing and some other preliminary processing procedures. The epidermis of citrus fruits (peel) consist of an epicuticular wax layer in platelets. The wax layer is deposited slowly during the development of the fruit; wax amount depends from species, from climate and from growing conditions. The fruit surface usually carries an assortment of dead and living fungi and bacteria, particularly if grown in humid climate; this accounts for the need of complete fruit cleaning before juice and oil winning to minimize any contamination coming from fruits surface. It is recommended to use a brusher - washer with water or with a disinfectant solution (e.g. chlorine 20 ppm). Immediately under epidermis is located the flavedo characterized by a green, yellow or orange colour, interspersed oil glands and no vascular bundle. oil glands are characterized by very thin and fragile walls, within essential oil is contained with a certain positive pressure and it affords oil recovery by abrasion of flavedo layer. Next layer is the albedo that is composed of loosely packed, many branched, tube-like cells that form a continuous network with the greatest part of tissue volume comprised of intercellular space. The albedo layer varies in thickness with the variety and it is a predominant factor in determining the proper setting of extractors when optimum quality is desired. Albedo is very rich in flavonoids, a class of chemicals which impart a bitter taste to the juice. Next layer is the endocarp, the edible part of the fruit with segments containing juice vesicles; the juice from a biosynthetic point of view is the liquid expressed from the cytoplasm and vacuoles of the internal cells within the vesicles. The most internal part is the core made by a spongy tissue similar to the albedo..
Citrus Chemical Composition
Carbohydrates represent most of soluble solids in citrus fruits; they are present both as simple sugars and as polysaccharides. Citrus soluble solids are on the average made by a 70 % of sugars, whilst pulp solids are made by a 40 % of sugars and by a 50 % of polysaccharides. Citrus flavor is due to the blend of sugars, acids (mainly citric acid and malic acid) and specific flavor compounds, some of which are sugar-containing substances known as glycosides. Contribution to fruit colour may be made by sugar-containing anthocyanidins while texture is controlled by the structural carbohydrate polymers.
Between monosaccharides, major components are glucose and fructose. Galactose is present just in some phenolic glycosides and in polysaccharides. There are not free pentose sugars, those compounds are present just in hemicelluloses and arabinans. 6-deoxyaldohexoses do not occur free, but fucose and rhamnose are constituents of citrus pectic substances. Saccharose is the main naturally occurring oligosaccharide in citrus fruits. Polysaccharides are mainly represented by pectic substances (galacturonans) (1-4) linked D-galactopiranuronic acid units in extended chains; their carboxyl group is partially or completely neutralized with one or more cations and some may be esterified by methanol to form esters (carbometoxy groups). Pectic Substances can be classified as follows:
- Protopectin : the term is applied to the water insoluble parent pectic substance which occurs in plants and which upon restricted hydrolysis yields pectin or pectinic acids.
- Pectinic Acids : the term is used for colloidal polygalacturonic acids containing more than a negligible portion of methyl ester groups. Pectinic acids, under suitable conditions, are capable of forming gels with sugar and acids or, if suitably low in methoxyl content, with certain metallic ions.
- Pectin : the term designates those water soluble pectinic acids of varying methyl ester content and degree of neutralization which are capable of forming gels with sugars and acids under suitable conditions.
- Pectic Acids : the term is applied to pectic substances mostly composed of colloidal polygalacturonic acids and essentially free from methyl ester groups.
Pectinic acids, the most useful of the pectic substances because of their jellying power, are divided into two groups of pectins for commercial gel making. High-methoxyl (above 7 % methoxyl) will form jams and jellies with the proper proportion of sugar and acids. The low-methoxyl pectins (3 to 7 % methoxyl) will form stable gels with small quantities of polyvalent cations such as calcium without the additional soluble solids and acids. Jellying power depends from the fruit used to make the pectin;, from the extraction system and from ripening degree of fruits. It is described as the proportion of sugars which one part of solid pectin or pectin extract is capable of turning, under prescribed conditions, into jelly of suitable characteristics. The best pectin is extracted from lemons. For all dispersed pectins in water, viscosity is dependent on concentration, pH, salts and size of the polygalacturonic acid chain. The higher the polymerization of the pectin molecule, the greater is its jelly grade; the lower is its methoxyl degree, the greater will be its jelly grade. Pectin changes during maturation, as the fruits ripen, the insoluble protopectin of meristematic and parenchymatous tissue changes into water-soluble pectin and pectinates; as the fruits continue to ripen and become over-ripe these products are converted into low grade-pectin and insoluble pectates, this is the reason why also juice and other by-products processing needs to be kept under control to prevent anomalous behaviors due to processing parameters control missing. Pectin changes may be enzymatic or chemical. Because enzyme are an important factor in the pectic changes that occur in citrus, only two pectic enzymes will be considered. Pectinesterase (PE) is an enzyme capable of demethylating pectin, and Polygalacturonase (PG) that is a pectic depolymerizing enzyme. Only PE naturally occurs in citrus, PG is used as technological adjuvant to decrease viscosity in concentrated juices and bases. PE is a very important factor to be taken in account during citrus product manufacturing. Its capacity of demethylating pectin causes viscosity increase that has to be controlled during orange juice evaporation for avoiding product gelation, moreover enzyme acts also on cloud stability and decrease turbidity up to complete clarification of the juice. Pectin, in fact, is the natural colloidal stabilizer of citrus juices, it is giving them their "body"; if it is affected by PE activity, juice clarifies and all suspended colloids will flock down. The best way for inactivating PE activity is heat. Pairs temperature-time to be used are depending from citrus species, from juice acidity and from its concentration degree. Generally speaking, higher is juice concentration, lower is the temperature needed for PE inactivation and higher is acidity, lower is the inactivation temperature. SO, for example, for a single strength orange juice are needed 99°C per 3 sec. or 88°C for 12 sec. whilst for a 65°Bx juice 71°C for 15 sec. are pretty enough; either for single strength lemon juice it is enough to use 74°C foe 122 sec or 88°C for 1 sec, whilst for very sweet mandarin juice you will need 91°C for 12 sec. or 99°C for 1 sec. PE activity is strictly linked to suspended solids, the so-called "pulp".
Let's consider now organic acids. They play a very important role in fruits growth and, also, in citrus products sales. Total acidity, together with total sugar content is an important criteria to evaluate the ripening degree of oranges and grapefruits whilst for lemon juice represents the primary factor for price definition. Acidity is, moreover, a critical point for consumer's acceptance; it would be impossible to trade an orange juice having grapefruit juice acidity, for instance. Organic acids in citrus are, basically, Citric Acid and L-Malic Acid that account for almost all the total acidity. D-Isocitric Acid is contained in small concentration, but is an important quality and purity criteria, whilst Oxalic Acid, Succinic Acid, Malonic Acid, Quinic Acid, Tartaric Acid, Adipic Acid, 2-ketoglutaric Acid are contained just in traces. Total acidity range has an extremely wide range between different species, but also growing area affect acidity. Mediterranean orange juices are more acid respect to ones produced in USA, in Brazil or Cuba. Organic acids are contained basically in the juice and their concentration in other fruits part is very low.
Nitrogenous Compound in Citrus are contained in rather small concentration but are to be considered important for a correct evaluation of juices purity. Between nitrogenous compounds the most important are free amminoacids that represent about 70 % of total nitrogenous compounds. Citrus juices contain almost all important amminoacids with the only exception of tryptophane. The most abundant are proline, asparagine, aspartic acid, serine, glutamic acid and arginine. Citrus contains small amounts of proteins which are, basically, enzymes (oxidoreductases, transferases, hydrolases and lyases, isomerases and ligases. Nitrogen bases and nucleic acids contents are extremely low.
Citrus lipids can be differentiated in three classes: nonpolar, polar (non ionic) and polar (ionic). Between nonpolar lipids we remember aldheydes, ketones and alcohols with long chain, carotins and their esters and some triglycerides. Non ionic polar lipids usually contain sugars like glycosilglycerides. Polar ionic lipids contain reactive functional groups like aminic, carboxylic or phosphoric; free fatty acids and phosphatidic acid belong to this group. Despite their small content, lipids are important because are involved in "off-flavors" development during juices storage. It is believed that undergoing to oxidative changes they develop hydroxyacids and others break-down products. Speaking about lipids we have to mention one of citrus by-products i.e. seed oil; any efficient seeds use has been always hindered by the difficulty to divide seeds from skins, segments and pulp. Moreover must be noted that a single citrus processing plant can't process in an economical way just its own seeds and a consortium plant should be considered. Dry citrus seeds contain from 28 to 35 % of oil that, not-refined has a pale-yellow colour and a smell recalling almonds. The oil contains 95 % tryglicerides and small amounts of free fatty acids, hydrocarbons, sterols, tocoferols, phospholipids, limonin. Main chemical characteristic is fatty acids distribution: the six mains are palmitic, palmitoleic, stearic, linoleic, oleic and linolenic; the ratio between saturated and not-saturated is between 1/3 and 1/5, like corn oil.
Carotenoids derive their name from the main representative of their group, beta-carotene and are pigments widely spread in nature responsible for bright shades ranging from yellow to deep red. Carotenoids are tetraterpenes formed by joining together of eight C5 isoprene units; the units are linked in a regular head to tail manner, except in the center of the molecule where the order is inverted tail to tail, so that the molecule is symmetrical.
From its basic structure, almost all others carotenoids can be formally derived by hydrogenation, cyclization, oxidation or any combination of these processes. From classification point of view, carotenoids can be divided in hydrocarbons C40H56 and their oxygenated derivatives, xanthofills which contain oxygen in form of epoxy - (5,6 and 5,8 epoxy), hydroxy - (monols, diols and poliols), keto - (oxo), methoxy - and carboxy groups. Other carotenoid groups are : the aromatic carotenoids with one or both end groups aromatic; allenic carotenoids with C=C=C grouping at one end of the central chain; and acetylenic carotenoids with triple link in 7,8 or 7',8'. The most important physiological role of carotenoids is to act as Vitamin A precursors in animal organism. Almost all animal species are able to enzymatically convert plant carotenoids of a special structure to Vitamin A. Beta-carotene, the most abundant provitamin supply two molecules of Vitamin A; other carotenoids with provitamin activity, and their number is limited, have the intact beta-ionone ring at only one-half of the molecule, e.g. alpha -carotene, gamma-carotene, 5,6-epoxy-beta-carotene, 5,8 -epoxy-beta-carotene, criptoxanthin and its monoepoxides and apo-beta-carotenals. Carotenoids are used as food colors and pigments. They are located in the plastids both of the flavedo and of the internal juice vesicles. In the early stages of fruit ripeness, external color is masked by chlorophyll; with advancing maturity the yellow color appears in various tints from light yellow to deep orange, due to the variations in type and amount of different carotenoids. The natural variation in the carotenoid level and type of components is very high. This variation depends on a multitude of factors such as environment, growing conditions, seasonal variations and stage of maturity. According to phenotipic interspecies differences, the overall carotenoid pattern in fruits varies from relatively simple mixtures to extremely complex ones.
A group of complex substances chemically related triterpene derivatives, has been named limonoids; all components have a furan ring attached to the D-ring at C-17. Between limonoids, the most important is limonin, known as citrus constituent since 1841. Limonin in not directly present in fruit tissues, it is contained as limonoic acid A-ring lactone thati it is not bitter. When the fruit is macerated, as happens when juice is squeezed, the combined action of fruits acid and an enzyme, converts this product to limonin, that is extremely bitter. In citrus products, this phenomenon is usually named "delayed bitterness". The absence of bitterness in the intact fruit and the delay in the onset of bitterness after juicing differentiates limonin bitterness from that due to the flavanone neohesperidosides, such as naringin of grapefruit or bitter orange. In this last cases, the intact fruit is bitter and the same is true for freshly squeezed juice. Flavanone neohesperidosides do not occur in many citrus species like sweet oranges (Citrus Sinensis), lemons (Citrus Limon), limes (Citrus Aurantifolia) and in mandarins and tangerines (Citrus Reticulata); limonin, on the other end, is ubiquitous in all citrus species, although it may not be present in sufficient quantity at maturity to cause delayed bitterness of the juice. Delayed bitterness is most noticeable in the juice of the Navel orange and the Shamouti orange. There is no way, after juicing, to stop reaction. There is a direct relationship between limonin concentration and bitterness; generally speaking, less than 6 ppm = not bitter; more than 9 ppm = bitter; 24 to 30 ppm = extremely bitter. Obviously, variations in acidity and sweetness influence subjective responses to bitterness, in fact a 1 ppm solution of limonin in water is already considered bitter.
We will end our survey on chemical composition of Citrus by treating of flavonoids. Flavonoids are very abundant in Citrus and have a very complex pattern. Three types of flavonoids occur in Citrus: flavanones (including 3-hydroxyflavanones), flavones (including 3-hydroxyflavones) and anthocyanins. Depending upon whether or not a glycosyl residue is present, the flavanones and flavones are further divided in O-glycosyl, aglycones and C-glycosylflavones. Anthocyanins are only known as constituents of blood oranges. The most important flavanones are hesperidin, naringin, poncirin, heriocitrin, neoheriocitrin and neohesperidin. Between flavones, the most important are rhoifolin, rutin and diosmin. Between aglycones, we remember sinensetin, auranetin, tangeritin. Don not taking in account anthocyanins, in citrus were found 56 flavonoids. Some of them are bitter and some show, like diosmin, hesperidin and rutin, pharmacological activity. In fact, Vitamin P, a very effective factor for reducing capillary permeability was discovered on 1937 by Szent and Gyoryi while working on lemon peels. Pharmacological use is important in vascular and trombotic diseases like varicose veins. Diosmin, in particular, reduces capillary damages induced by histamine. Often crude flavonoids extracts are employed in drugs formulas. Recently has been discovered that some flavonoids acts as regulators in prostaglandins synthesis, a class of natural compounds physiologically very active action on different types of muscles and involved in inflammatory states, in platelets aggregation and in others physiological processes. Some flavonoids act as inhibitors and some others as stimulators of enzymatic complex of those substances biosynthesis. Some flavonoids, like hydroxyethylrutinosides are anti-inflammatory, other are immunosuppressive and other show antiallergenic properties. Recently has been discovered an anticancerous activity of tangeritin that seems able to delay metastasis trough a primary cancer change to secondary and tertiary stages. Were studied also hesperidin, naringin and nobiletin that have shown much lower capacities. Moreover, dihydrochalcones derivatives of flavonoids are low calories sweeteners. Flavonoids are located mainly in the albedo and their concentration in juices depends strictly by juicing technology. Due to their bitterness, it is desiderable to remove from juices (like for limonin) and this can be obtained through absorber resins. With respect to anthocyanins, the most abundant is cyanidin-3-glucoside, then cyanidina-3,5-diglucoside, peonidin-5-glucoside, delphinidin-3-glucoside and petunidin-3-glucoside.
Nitrogenous Compound in Citrus are contained in rather small concentration but are to be considered important for a correct evaluation of juices purity. Between nitrogenous compounds the most important are free amminoacids that represent about 70 % of total nitrogenous compounds. Citrus juices contain almost all important amminoacids with the only exception of tryptophane. The most abundant are proline, asparagine, aspartic acid, serine, glutamic acid and arginine. Citrus contains small amounts of proteins which are, basically, enzymes (oxidoreductases, transferases, hydrolases and lyases, isomerases and ligases. Nitrogen bases and nucleic acids contents are extremely low.
Citrus lipids can be differentiated in three classes: nonpolar, polar (non ionic) and polar (ionic). Between nonpolar lipids we remember aldheydes, ketones and alcohols with long chain, carotins and their esters and some triglycerides. Non ionic polar lipids usually contain sugars like glycosilglycerides. Polar ionic lipids contain reactive functional groups like aminic, carboxylic or phosphoric; free fatty acids and phosphatidic acid belong to this group. Despite their small content, lipids are important because are involved in "off-flavors" development during juices storage. It is believed that undergoing to oxidative changes they develop hydroxyacids and others break-down products. Speaking about lipids we have to mention one of citrus by-products i.e. seed oil; any efficient seeds use has been always hindered by the difficulty to divide seeds from skins, segments and pulp. Moreover must be noted that a single citrus processing plant can't process in an economical way just its own seeds and a consortium plant should be considered. Dry citrus seeds contain from 28 to 35 % of oil that, not-refined has a pale-yellow colour and a smell recalling almonds. The oil contains 95 % tryglicerides and small amounts of free fatty acids, hydrocarbons, sterols, tocoferols, phospholipids, limonin. Main chemical characteristic is fatty acids distribution: the six mains are palmitic, palmitoleic, stearic, linoleic, oleic and linolenic; the ratio between saturated and not-saturated is between 1/3 and 1/5, like corn oil.
Carotenoids derive their name from the main representative of their group, beta-carotene and are pigments widely spread in nature responsible for bright shades ranging from yellow to deep red. Carotenoids are tetraterpenes formed by joining together of eight C5 isoprene units; the units are linked in a regular head to tail manner, except in the center of the molecule where the order is inverted tail to tail, so that the molecule is symmetrical.
From its basic structure, almost all others carotenoids can be formally derived by hydrogenation, cyclization, oxidation or any combination of these processes. From classification point of view, carotenoids can be divided in hydrocarbons C40H56 and their oxygenated derivatives, xanthofills which contain oxygen in form of epoxy - (5,6 and 5,8 epoxy), hydroxy - (monols, diols and poliols), keto - (oxo), methoxy - and carboxy groups. Other carotenoid groups are : the aromatic carotenoids with one or both end groups aromatic; allenic carotenoids with C=C=C grouping at one end of the central chain; and acetylenic carotenoids with triple link in 7,8 or 7',8'. The most important physiological role of carotenoids is to act as Vitamin A precursors in animal organism. Almost all animal species are able to enzymatically convert plant carotenoids of a special structure to Vitamin A. Beta-carotene, the most abundant provitamin supply two molecules of Vitamin A; other carotenoids with provitamin activity, and their number is limited, have the intact beta-ionone ring at only one-half of the molecule, e.g. alpha -carotene, gamma-carotene, 5,6-epoxy-beta-carotene, 5,8 -epoxy-beta-carotene, criptoxanthin and its monoepoxides and apo-beta-carotenals. Carotenoids are used as food colors and pigments. They are located in the plastids both of the flavedo and of the internal juice vesicles. In the early stages of fruit ripeness, external color is masked by chlorophyll; with advancing maturity the yellow color appears in various tints from light yellow to deep orange, due to the variations in type and amount of different carotenoids. The natural variation in the carotenoid level and type of components is very high. This variation depends on a multitude of factors such as environment, growing conditions, seasonal variations and stage of maturity. According to phenotipic interspecies differences, the overall carotenoid pattern in fruits varies from relatively simple mixtures to extremely complex ones.
A group of complex substances chemically related triterpene derivatives, has been named limonoids; all components have a furan ring attached to the D-ring at C-17. Between limonoids, the most important is limonin, known as citrus constituent since 1841. Limonin in not directly present in fruit tissues, it is contained as limonoic acid A-ring lactone thati it is not bitter. When the fruit is macerated, as happens when juice is squeezed, the combined action of fruits acid and an enzyme, converts this product to limonin, that is extremely bitter. In citrus products, this phenomenon is usually named "delayed bitterness". The absence of bitterness in the intact fruit and the delay in the onset of bitterness after juicing differentiates limonin bitterness from that due to the flavanone neohesperidosides, such as naringin of grapefruit or bitter orange. In this last cases, the intact fruit is bitter and the same is true for freshly squeezed juice. Flavanone neohesperidosides do not occur in many citrus species like sweet oranges (Citrus Sinensis), lemons (Citrus Limon), limes (Citrus Aurantifolia) and in mandarins and tangerines (Citrus Reticulata); limonin, on the other end, is ubiquitous in all citrus species, although it may not be present in sufficient quantity at maturity to cause delayed bitterness of the juice. Delayed bitterness is most noticeable in the juice of the Navel orange and the Shamouti orange. There is no way, after juicing, to stop reaction. There is a direct relationship between limonin concentration and bitterness; generally speaking, less than 6 ppm = not bitter; more than 9 ppm = bitter; 24 to 30 ppm = extremely bitter. Obviously, variations in acidity and sweetness influence subjective responses to bitterness, in fact a 1 ppm solution of limonin in water is already considered bitter.
We will end our survey on chemical composition of Citrus by treating of flavonoids. Flavonoids are very abundant in Citrus and have a very complex pattern. Three types of flavonoids occur in Citrus: flavanones (including 3-hydroxyflavanones), flavones (including 3-hydroxyflavones) and anthocyanins. Depending upon whether or not a glycosyl residue is present, the flavanones and flavones are further divided in O-glycosyl, aglycones and C-glycosylflavones. Anthocyanins are only known as constituents of blood oranges. The most important flavanones are hesperidin, naringin, poncirin, heriocitrin, neoheriocitrin and neohesperidin. Between flavones, the most important are rhoifolin, rutin and diosmin. Between aglycones, we remember sinensetin, auranetin, tangeritin. Don not taking in account anthocyanins, in citrus were found 56 flavonoids. Some of them are bitter and some show, like diosmin, hesperidin and rutin, pharmacological activity. In fact, Vitamin P, a very effective factor for reducing capillary permeability was discovered on 1937 by Szent and Gyoryi while working on lemon peels. Pharmacological use is important in vascular and trombotic diseases like varicose veins. Diosmin, in particular, reduces capillary damages induced by histamine. Often crude flavonoids extracts are employed in drugs formulas. Recently has been discovered that some flavonoids acts as regulators in prostaglandins synthesis, a class of natural compounds physiologically very active action on different types of muscles and involved in inflammatory states, in platelets aggregation and in others physiological processes. Some flavonoids act as inhibitors and some others as stimulators of enzymatic complex of those substances biosynthesis. Some flavonoids, like hydroxyethylrutinosides are anti-inflammatory, other are immunosuppressive and other show antiallergenic properties. Recently has been discovered an anticancerous activity of tangeritin that seems able to delay metastasis trough a primary cancer change to secondary and tertiary stages. Were studied also hesperidin, naringin and nobiletin that have shown much lower capacities. Moreover, dihydrochalcones derivatives of flavonoids are low calories sweeteners. Flavonoids are located mainly in the albedo and their concentration in juices depends strictly by juicing technology. Due to their bitterness, it is desiderable to remove from juices (like for limonin) and this can be obtained through absorber resins. With respect to anthocyanins, the most abundant is cyanidin-3-glucoside, then cyanidina-3,5-diglucoside, peonidin-5-glucoside, delphinidin-3-glucoside and petunidin-3-glucoside.
The inorganic elements of citrus fruits and products comprise the material (ash) remaining after all organic matter has been destroyed. The percentage of ash and the relative concentrations of inorganic constituents are dependent upon growing conditions (fertilization, soil type, climate, temperature and rainfall), tree health, cultivars, stage of maturity, season of harvest and geographic origin. Likewise, the percentage distribution of inorganic elements in processed products is dependent upon several processing parameters like pressure used to juice fruit, pulp control, finishing and pulp washing. Main inorganics are potassium, phosphor, calcium and magnesium, with lower concentration are contained sodium, chlorine, nitrogen and iron.
With respect to vitamins, the most important is Vitamin C (Ascorbic acid), a glass of orange juice supply 60 & of RDA, other vitamins are : Pholacine, Vitamin B6, Thiamine, Riboflavin, Biotin, Pantotenic acid and products with Vitamin A type activity. Average quantities in freshly sqeezed orange juice are reporten in the following table:
With respect to vitamins, the most important is Vitamin C (Ascorbic acid), a glass of orange juice supply 60 & of RDA, other vitamins are : Pholacine, Vitamin B6, Thiamine, Riboflavin, Biotin, Pantotenic acid and products with Vitamin A type activity. Average quantities in freshly sqeezed orange juice are reporten in the following table:
| Vitamina / Vitamin | Unità/100 ml | |
|---|---|---|
| Acido Ascorbico / Ascorbic Acid | mg | 35 - 56 |
| Tiamina / Thiamine | mg | 60 - 145 |
| Ribiflavina / Ribophlavin | mg | 11 - 90 |
| Niacina / Nyacin | mg | 200 - 300 |
| B6 | mg | 25 - 80 |
| Folacina / Folacin | mg | 120 - 330 |
| Acido Pantotenico / Pantotenic Acid | mg | 130 - 210 |
| Biotina / Biotin | mg | 1 - 3 |
| Vitamina A (attività) | IU | 190 - 400 |
Analisi Succhi di agrumi / Citrus Juices Analysis
Succo di Arancia / Orange Juice
Valori RSK
Valore Value | Standard | Range | Media Average | ||
|---|---|---|---|---|---|
| min. | 1,045 | 1,045 - 1,055 | 1,046 | Densità Relativa 20°C / Relative Density | |
| min. | 11,18 | 11,18 - 13,54 | 11,41 | °Brix Rifrat. corr. | |
| meq/l | 90 - 240 | Acidità Titolab. pH 8,1 / Titratable Acidity | |||
| g/l | max. | 3,0 | Etanolo / Ethanol | ||
| g/l | max. | 0,4 | Acidità Volatile (come ac. acetico) Volatile Acids (as acetic acid) | ||
| g/l | max. | 0,5 | Acido lattico / Lactin Acid | ||
| mg/l | min. | 200 | 350 | Acido L-Ascorbico / Ascorbic Acid | |
| g/l | max. | 0,3 | Oli Essenziali / Volatile Oils | ||
| g/l | min. | 22 | 20 - | 28 | Glucosio /Glucose |
| g/l | min. | 24 | 22 - | 30 | Fruttosio / Fructose |
| max. | 1,0 | 0,85 - 1,0 | 0,92 | Glucosio/Fruttosio - Glucose/Fructose | |
| g/l | max. | 45 | - 47 | 33 | Saccarosio / Sucrose |
| max. | 50 | Saccarosio % su zuccheri totali Sucrose % on total sugars | |||
| g/l | min. | 26 | 24 - 37 | 28 | Estratto Non Zuccherino / Sugarfree Extract |
| g/l | min. | 3,5 | 2,9 - 4,8 | 4,0 | Ceneri / Ash |
| min. | 11,0 | 10,5 - 14,0 | 12,5 | Alcalinità delle Ceneri / Ash Alcalinity | |
| mg/ | max. | 30 | 14 | Sodio (Na) / Sodium | |
| mg/l | min. | 1700 | 1400 - 2300 | 1900 | Potassio (K) / Potassium |
| mg/l | max. | 110 | 60 - 120 | 80 | Calcio (Ca) / Calcium |
| mg/l | min. | 90 | 70 - 150 | 100 | Magnesio (Mg) / Magnesium |
| mg/l | max. | 60 | Cloruri (Cl) / Chloride | ||
| mg/l | max. | 10 | Nitrati (NO3) / Nitrates | ||
| mg/l | min. | 400 | 350 - 600 | 460 | Fosfati (PO4) / Phosphates |
| mg/l | max. | 150 | Solfati (SO4) / Sulphates | ||
| g/l | min. | 8,0 | 7,6 - 11,5 | 9,4 | Acido Citrico / Citric Acid |
| mg/l | min. | 70 | 65 - 130 | 90 | Acido Isocitrico / Isocitric Acid |
| max. | 130 | 80 - 130 | 105 | Citrico/Isocitrico / Citric/Isocitric | |
| g/l | max. | 2,5 | 1,1 - 2,9 | 1,7 | Acido L-Malico / Malic Acid |
| mg/l | min. | 575 | 450 - 1300 | 800 | Prolina / Proline |
| min. | 18 | 15 - 26 | 20 | Nr. Formolo (ml NaOH 0,1N/100 ml) Formol Number | |
| mg/l | max. | 1000 | 500 - 1000 | 800 | Flavonoidi Totali (Davis) come esperidina Hesperidin (Davis) |
| mg/l | max. | 500 | 300 | Pectine Solubili (come ac. galatturonico) Water Soluble Pectins (as galacturonic acid) | |
| mg/l | max. | 200 | Pectine ossalato solubili (come ac. galatt.) Oxalate soluble pectins (as galacturonic ac.) | ||
| mg/l | max | 300 | Pectine alcali solubili (come ac. galatt.) Alkali soluble pectins (as galacturonic ac.) | ||
| mg/l | max. | 15 | Carotenoidi Totali 7 Total Carotenoids | ||
| max. | 5 | beta-carotene (% su carotenoidi tot.) beta-carotene (% on total carotenoids) | |||
| max. | 15 | Criptoxantine (% su carotenoidi tot.) Cryptoxanthins (% on total caroten.) | |||
| Analisi Amminoacidi / Aminoacid Analysis | |||||
| mg/l | 225 - 400 | 380 | Acido Aspartico / Aspartic acid | ||
| mg/l | 12 - 36 | 19 | Treonina / Threonine | ||
| mg/l | 105 - 189 | 164 | Serina / Serine | ||
| mg/l | 255 - 675 | 450 | Asparagina / Asparagine | ||
| mg/l | 73 - 162 | 118 | Acido Glutammico / Glutamic Acid | ||
| mg/l | max. | 73 | Glutammina / Glutamine | ||
| mg/l | 450 - 1300 | 800 | Prolina / Proline | ||
| mg/l | 11 - 23 | 15 | Glicina / Glycine | ||
| mg/l | 63 - 135 | 99 | Alanina / Alanine | ||
| mg/l | 8 - 27 | 18 | Valina /Valine | ||
| mg/l | max. | 5 | Metionina / Methionine | ||
| mg/l | 3 - 9 | 6 | Leucina / Leucine | ||
| mg/l | 2,6 - 8 | 5 | Isoleucina / Isoleucine | ||
| mg/l | 4 - 18 | 9 | Tirosina / Tyrosine | ||
| mg/l | 13 - 50 | 30 | Fenilalanina / Phenylalanine | ||
| mg/l | 175 - 360 | 240 | Acido gamma-amminobutirrico gamma-aminobutirric acid | ||
| mg/l | 3 - 13 | 8 | Ornitina / Ornithine | ||
| mg/l | 22 - 58 | 29 | Lisina / Lysine | ||
| mg/l | 5 - 19 | 11 | Istidina /Histidine | ||
| mg/l | 435 - 1050 | 700 | Arginina / Arginin | ||
| mg/l | max. | 26 | Ammoniaca / Ammonia | ||
| mg/l | max. | 37 | Etanolammina / Ethanolamine |
Analisi Succhi di agrumi - Valori AIJNCitrus Juices Analysis - AIJN Values
Succo di Arancia / Orange Juice
Valore Value | Standard | Range | |||
|---|---|---|---|---|---|
| min. | 1,045 | Densità Relativa 20°C / Relative Density | |||
| min. | 11,2 | °Brix Rifrat. corr. | |||
| meq/l | 90 - 240 | Acidità Titolabile pH 8,1 / Titratable Acidity | |||
| g/l | max. | 3,0 | Etanolo / Ethanol | ||
| g/l | max. | 0,4 | Acidità Volatile (come ac. acetico) Volatile Acids (as acetic acid) | ||
| g/l | max. | 0,2 | Acido lattico / Lactic acid | ||
| mg/l | min. | 200 | Acido L-Ascorbico / Ascorbic Acid | ||
| g/l | 6,3 - 17 | Acido Citrico (enzimatico) / Citric acid | |||
| g/l | 20 - 35 | Glucosio / Glucose | |||
| g/l | 20 - 35 | Fruttosio / Fructose | |||
0,85 - 1,0 | Glucosio/Fruttosio - Glucose/Fructose | ||||
| g/l | 10 - 50 | Saccarosio / Sucrose | |||
| mg/l | max. | 10 | Idrossimetilfurfurolo Hydroxymethylfurfural (HMF) | ||
| g/l | 24 - 40 | Estratto Non Zuccherino / Sugarfree xtract | |||
| g/l | 2,2 - 4,3 | Ceneri / Ash | |||
| mg/l | Assente | Acido D-Malico / D.Malic Acid | |||
| mg/l | max. | 30 | Sodio (Na) / Sodium | ||
| mg/l | 1300 - 2500 | Potassio (K) / Potassium | |||
| mg/l | 50 - 160 | Calcio (Ca) / Calcium | |||
| mg/l | 70 - 160 | Magnesio (Mg) / Magnesium | |||
| mg/l | 115 - 210 | Fosforo Totale (P) / Total Phosphorous | |||
| mg/l | max. | 120 | Solfati (SO4) / Sulphates | ||
| mg/l | max. | 5 | Nitrati (NO3) /Nitrates | ||
| mg/l | 65 - 200 | Acido Isocitrico / Isocitric Acid | |||
| mg/l | max. | 130 | Citrico/Isocitrico - Citric/Isocitric | ||
| g/l | 0,8 - 3,0 | Acido L-Malico / L-Malic acid | |||
15 - 26 | Nr. Formolo (ml NaOH 0,1N/100 ml) Formol Number | ||||
| mg/l | 250 - 700 | Esperidina (Davis) / Hesperidin | |||
| mg/l | max. | 500 | Pectine Solubili (come ac. galatturonico) Water Soluble Pectin (as galacturonic acid) | ||
| Valori isotopici / Isotopic Values | |||||
(-27) - (-24) (-28) - (-25) (-28) - (-23,5) (-25,5 - (-22,5) (-25,5) - (-22,5) | |||||
| ppm | 103 - 107 | (D/H) Etanolo (Ethanol) D-NMR | |||
| min. | 0 | ||||
| mg/l | max. | 15 | Carotenoidi Totali / Total Carotenoids | ||
| % | max. | 5 | on tot. carot. | Idrocarburi Carotenici Carotenoid Hydrocarbons | |
| % | max. | 15 | on tot. carot. | Esteri Carotenici Carotenoid Esters | |
| % | max. | 15 | on tot. carot. | Xantofille / Xantophylester | |
| Analisi amminoacidi / Aminoacids Analysis | |||||
| mg/l | 200 - 400 | Acido Aspartico / Aspartic acid | |||
| mg/l | 10 - 50 | Treonina / Threonine | |||
| mg/l | 105 - 210 | Serina / Serine | |||
| mg/l | 225 - 660 | Asparagina / Asparagine | |||
| mg/l | 75 - 205 | Acido Glutammico / Glutamic acid | |||
| mg/l | max. | 75 | Glutammina / Glutamine | ||
| mg/l | 450 - 2090 | Prolina / Proline | |||
| mg/l | 10 - 25 | Glicina / Glycine | |||
| mg/l | 60 - 205 | Alanina / Alanine | |||
| mg/l | 10 - 30 | Valina / Valine | |||
| mg/l | max. | 5 | Metionina / Methionine | ||
| mg/l | 3 - 15 | Leucina / Leucine | |||
| mg/l | 3 - 15 | Isoleucina / Isoleucine | |||
| mg/l | 5 - 20 | Tirosina / Tyrosine | |||
| mg/l | 15 - 55 | Fenilalanina / Phenylalanine | |||
| mg/l | 180 - 500 | Acido gamma-amminobutirrico Gamma-aminobutirric Acid | |||
| mg/l | 3 - 20 | Ornitina / Ornithine | |||
| mg/l | 20 - 65 | Lisina / Lysine | |||
| mg/l | 5 - 25 | Istidina / Hystidine | |||
| mg/l | 400 - 1000 | Arginina / Arginin | |||
| mg/l | max. | 25,5 | Ammoniaca / Ammonia | ||
| mg/l | max. | 36,6 | Etanolammina / Ethanolamine | ||
| Metalli Pesanti / Heavy metals | |||||
| mg/kg | max. | 0,1 | Arsenico (As) / Arsenic | ||
| mg/kg | max. | 0,05 | Piombo (Pb) / Lead | ||
| mg/kg | max. | 0,01 | Mercurio (Hg) / Mercury | ||
| mg/kg | max. | 0,05 | Cadmio (Cd) / Cadmium | ||
| mg/kg | max. | 1,0 | Stagno (Sn) / Tin | ||
| mg/kg | max. | 5,0 | Rame (Cu) / Copper | ||
| mg/kg | max. | 5,0 | Zinco (Zn) / Zinc | ||
| mg/kg | max. | 5,0 | Ferro (Fe) / Iron |
Analisi Succhi di agrumi - Valori AIJNCitrus Juices Analysis - AIJN Values
Succo di Limone / Lemon Juice
Valore Value | Standard | Range | |||
|---|---|---|---|---|---|
| min. | 1,032 | Densità Relativa 20°C / Relative Density | |||
| min. | 8,0 | °Brix Rifrat. corr. | |||
| meq/l | 700 - 970 | Acidità Titolabile pH 8,1 Titratable Acidity | |||
| g/l | max. | 3,0 | Etanolo / Ethanol | ||
| g/l | max. | 0,4 | Acidità Volatile (come ac. acetico) Volatile Acids (as acetic acid) | ||
| g/l | max. | 0,2 | Acido lattico / Lactic Acid | ||
| mg/l | min. | 150 | Acido Ascorbico / Ascorbic Acid | ||
| g/l | 45 - 63 | Acido Citrico (enzimatico) / Citric acid | |||
| g/l | 3 - 12 | Glucosio / Glucose | |||
| g/l | 3 - 11 | Fruttosio / Fructose | |||
0,95 - 1,3 | Glucosio/Fruttosio - Glucose/Fructose | ||||
| g/l | max. | 7 | Saccarosio / Sucrose | ||
| mg/l ml/l | max. max. | 20 0,5 | Idrossimetilfurfurolo / Hydroxymethylfurfural (HMF) Oli Essenziali / Volatile Oils | ||
| g/l | 65 - 82 | Estratto Non Zuccherino / Sugarfree Extract | |||
| g/l | 2,2 - 4,3 | Ceneri / Ash | |||
13 - 26 | Numero di Formolo (ml NaOH 0,1N/100 ml) Formol Number | ||||
| mg/l | max. | 30 | Sodio (Na) / Sodium | ||
| mg/l | 1100 - 2000 | Potassio (K) / Potassium | |||
| mg/l | 45 - 160 | Calcio (Ca) / Calcium | |||
| mg/l | 70 - 120 | Magnesio (Mg) / Magnesium | |||
| mg/l | 80 - 150 | Fosforo Totale (P) / Total Phosphorous | |||
| mg/l | max. | 100 | Solfati (SO4) / Sulphates | ||
| mg/l | max. | 5 | Nitrati (NO3) / Nitrates | ||
| mg/l | 230 - 500 | Acido Isocitrico / Isocitric Acid | |||
| mg/l | max. | 200 | Citrico/Isocitrico - Citric/Isocitric | ||
| g/l | 1,0 - 7,5 | Acido L-Malico / L-Malic acid | |||
| mg/l | max. | 1500 | Esperidina (Davis) / Hesperidin | ||
| mg/l | max. | 700 | Pectine Solubili (come ac. galatturonico) Water Soluble Pectin (as galacturonic acid) | ||
| Valori isotopici / Isotopic Values | |||||
| | (-27) - (-24) | ||||
| min. | 15 | ||||
| min. | 0 | ||||
mg/kg | max. | 0,1 | Metalli Pesanti / Heavy Metals Arsenico (As) / Arsenic | ||
| mag/kg | max. | 0,05 | Piombo (Pb) / Lead | ||
| mg/kg mg/kg | max. max. | 5,0 5,0 | Rame (Cu) / Copper Zinco (Zn) / Zinc | ||
| mg/kg mg/kg | max. max. | 5,0 1,0 | Ferro (Fe) / Iron Stagno (Sn) / Tin | ||
| mg/kg | max. | 0,01 | Mercurio (Hg) / Mercury | ||
| mg/kg | max. | 0,02 | Cadmio (Cd) / Cadmium | ||
mg/l | 300 - 800 | Analisi Amminoacidi / Aminoacids Analysis Acido Aspartico / Aspartic Acid | |||
| mg/l | 10 - 30 | Treonina / Threonine | |||
| mg/l | 135 - 370 | Serina / Serine | |||
| mg/l | 130 - 600 | Asparagina / Asparagine | |||
| mg/l | 160 - 400 | Acido Glutammico / Glutamic Acid | |||
| mg/l | max. | 45 | Glutammina / Glutamine | ||
| mg/l | 100 - 800 | Prolina / Proline | |||
| mg/l | 7 - 25 | Glicina / Glycine | |||
| mg/l | 80 - 260 | Alanina / Alanine | |||
| mg/l | 8 - 35 | Valina / Valine | |||
| mg/l | max. | 5 | Metionina / Methionine | ||
| mg/l | 3 - 10 | Leucina / Leucine | |||
| mg/l | 3 - 10 | Isoleucina / Isoleucine | |||
| mg/l | max. | 7 | Tirosina / Tyrosine | ||
| mg/l | 8 - 40 | Fenilalanina / Phenylalanine | |||
| mg/l | 60 - 185 | Acido gamma-amminobutirrico gamma-aminobutirric Acid | |||
| mg/l | max. | 5 | Ornitina / Ornithine | ||
| mg/l | 5 - 20 | Lisina / Lysine | |||
| mg/l | max. | 10 | Istidina / Hystidine | ||
| mg/l | max. | 100 | Arginina / Arginin | ||
| mg/l | max. | 100 | Ammoniaca / Ammonia | ||
| mg/l | max. | 30 | Etanolammina / Ethanolamine |
Analisi Succhi di agrumi - Valori AIJNCitrus Juices Analysis - AIJN Values
Succo di Pompelmo / Grapefruit Juice
Valore Value | Standard | Range | |||
|---|---|---|---|---|---|
| min. | 1,040 | Densità Relativa 20°C Relative Density | |||
| min. | 10,0 | °Brix Rifrat. corr. | |||
| meq/l | 120 - 290 | Acidità Titolabile pH 8,1 Titratable Acidity | |||
| g/l | max. | 3,0 | Etanolo / Ethanol | ||
| g/l | max. | 0,4 | come ac. acetico as acetic acid | Acidità Volatile Volatile Acids | |
| g/l | max. | 0,2 | Acido lattico / Lactic acid | ||
| mg/l | min. | 200 | Acido L-Ascorbico / Ascorbic Acid | ||
| g/l | 8 - 20 | Acido Citrico (enzimatico) Citric acid | |||
| g/l | 20 - 50 | Glucosio / Glucose | |||
| g/l | 20 - 50 | Fruttosio / Fructose | |||
| max. | 1,02 | Glucosio/Fruttosio- Glucose/Fructose | |||
| g/l | 5 . 40 | Saccarosio / Sucrose | |||
| mg/l ml/l | max. max. | 10 0,3 | (HMF) | Idrossimetilfurfurolo Hydroxymethylfurfural Olio Essenziale / Volatile Oils | |
| g/l | 25 - 40 | Estratto Non Zuccherino Sugarfree Extract | |||
| g/l | 2,3 - 4,5 | Ceneri / Ash | |||
| ml NaOH 0,1N/100 ml | 14 - 30 | Numero di Formolo / Formol Number | |||
| mg/l | max. | 30 | Sodio (Na) / Sodium | ||
| mg/l | 900 - 2000 | Potassio (K) / Potassium | |||
| mg/l | 50 - 160 | Calcio (Ca) / Calcium | |||
| mg/l | 65 - 150 | Magnesio (Mg) / Magnesium | |||
| mg/l | 100 - 200 | Fosforo Totale (P) Total Phosphorous | |||
| mg/l | max. | 150 | Solfati (SO4) / Sulphates | ||
| mg/l | max. | 5 | Nitrati (NO3) / Nitrates | ||
| mg/l | 140 - 350 | Acido Isocitrico / Isocitric Acid | |||
| mg/l | max. | 200 | Citrico/Isocitrico - Citric/Isocitric | ||
| g/l | 0,2 - 1,2 | Acido L-Malico / L-Malic Acid | |||
| mg/l | max. | 1200 | Naringina (Davis) / Naringin | ||
| mg/l | max. | 500 | come ac. galatturonico as galacturonic acid | Pectine Solubili ( Water Soluble Pectin | |
| Valori Isotopici / Isotopic Values | |||||
ppm | (-28) - (-25) (-29) - +(29) (-28) - (-24,5) (-26,5) - (-23,5) 102-106 | (D/H) Etanolo (ethanol) D-NMR | |||
| min. | 15 | ||||
| min. | 0 | ||||
mg/kg | max. | 0,1 | Metalli Pesanti / Heavy metals Arsenico (As) / Arsenic | ||
| mag/kg | max. | 0,05 | Piombo (Pb) / Lead | ||
| mg/kg mg/kg | max. max. | 5,0 5,0 | Rame (Cu) / Copper Zinco (Zn) / Zinc | ||
| mg/kg mg/kg | max. max. | 5,0 1,0 | Ferro (Fe) / Iron Stagno (Sn) / Tin | ||
| mg/kg | max. | 0,01 | Mercurio (Hg) mercury | ||
| mg/kg | max. | 0,02 | Cadmio (Cd) / Cadmium | ||
mg/l | 400 - 800 | Analisi Amminoacidi / Aminoacid Analysis Acido Aspartico / Aspartic Acid | |||
| mg/l | 12 - 36 | Treonina / Threonine | |||
| mg/l | 105 - 210 | Serina / Serine | |||
| mg/l | 240 - 800 | Asparagina / Asparagine | |||
| mg/l | 80 - 235 | Acido Glutammico / Glutamic Acid | |||
| mg/l | max. | 75 | Glutammina / Glutamine | ||
| mg/l | 200 - 1400 | Prolina / Proline | |||
| mg/l | 11 - 38 | Glicina Glycine | |||
| mg/l | 62 - 180 | Alanina / Alanine | |||
| mg/l | 12 - 35 | Valina / Valine | |||
| mg/l | max. | 10 | Metionina / Methionine | ||
| mg/l | 1 - 10 | Leucina / Leucine | |||
| mg/l | 1 - 10 | Isoleucina / Isoleucine | |||
| mg/l | max. | 18 | Tirosina / Tyrosine | ||
| mg/l | 9 - 46 | Fenilalanina / Phenylalanine | |||
| mg/l | 180 - 570 | Acido gamma-amminobutirrico gamma-aminobutirric acid | |||
| mg/l | 1 - 26 | Ornitina / Ornithine | |||
| mg/l | 12 - 58 | Lisina / Lysine | |||
| mg/l | 2 - 25 | Istidina / Hystidine | |||
| mg/l | 240 - 830 | Arginina / Arginin | |||
| mg/l | 14 - 50 | Ammoniaca / Ammonia | |||
| mg/l | max. | 24,4 | Etanolammina / Ethanolamine |
Dati Analitici Oli EssenzialiEssential Oils Analysis
| Olio di Arancia - Orange Oil | Min. | Max. |
|---|---|---|
| Densità Relativa 20°C - Relative Density 20°C | 0,844 | 0,847 |
| Indice di Rifrazione 20°C - Refractive Index 20°C | 1,473 | 1,475 |
| Potere Rotatorio 20°C - Optical Rotation 20°C | +97 | +99 |
| Residuo all'Evaporazione - Evaporation Residue (%) | 1,6 | 3,5 |
| Aldeidi (% come aldeide decilica) - Aldheydes (% as decanal) | 0,9 | 2,2 |
.
Dati Analitici Oli EssenzialiEssential Oils Analysis
| Olio di Limone - Lemon Oil | Min. | Max. |
|---|---|---|
| Densità Relativa 20°C - Relative Density 20°C | 0,849 | 0,858 |
| Indice di Rifrazione 20°C - Refractive Index 20°C | 1,474 | 1,476 |
| Potere Rotatorio 20°C - Optical Rotation 20°C | +57 | +65 |
| Residuo all'Evaporazione - Evaporation Residue (%) | 1,6 | 3,9 |
| Aldeidi (% come citral) - Aldheydes (% as citral) | 3,33 | 4,68 |
| CD (250 mg/100 ml etanolo (ethanol) 95% V/V | 0,450 | 0,950 |
.
Dati Analitici Oli EssenzialiEssential Oils Analysis
| Olio di Mandarino- Mandarin Oil | Min. | Max. |
|---|---|---|
| Densità Relativa 20°C - Relative Density 20°C | 0,850 | 0,855 |
| Indice di Rifrazione 20°C - Refractive Index 20°C | 1,473 | 1,477 |
| Potere Rotatorio 20°C - Optical Rotation 20°C | +64 | +75 |
| Residuo all'Evaporazione - Evaporation Residue (%) | 2,0 | 4,0 |
| Aldeidi (% come aldeide decilica) - Aldheydes (% as decanal) | 0,4 | 1,2 |
| CD (150 mg/100 ml etanolo (ethanol) 95% V/V | 0,500 | |
| Max 323 +/- 3 nm | ||
.